Why Buy Authorized Generics
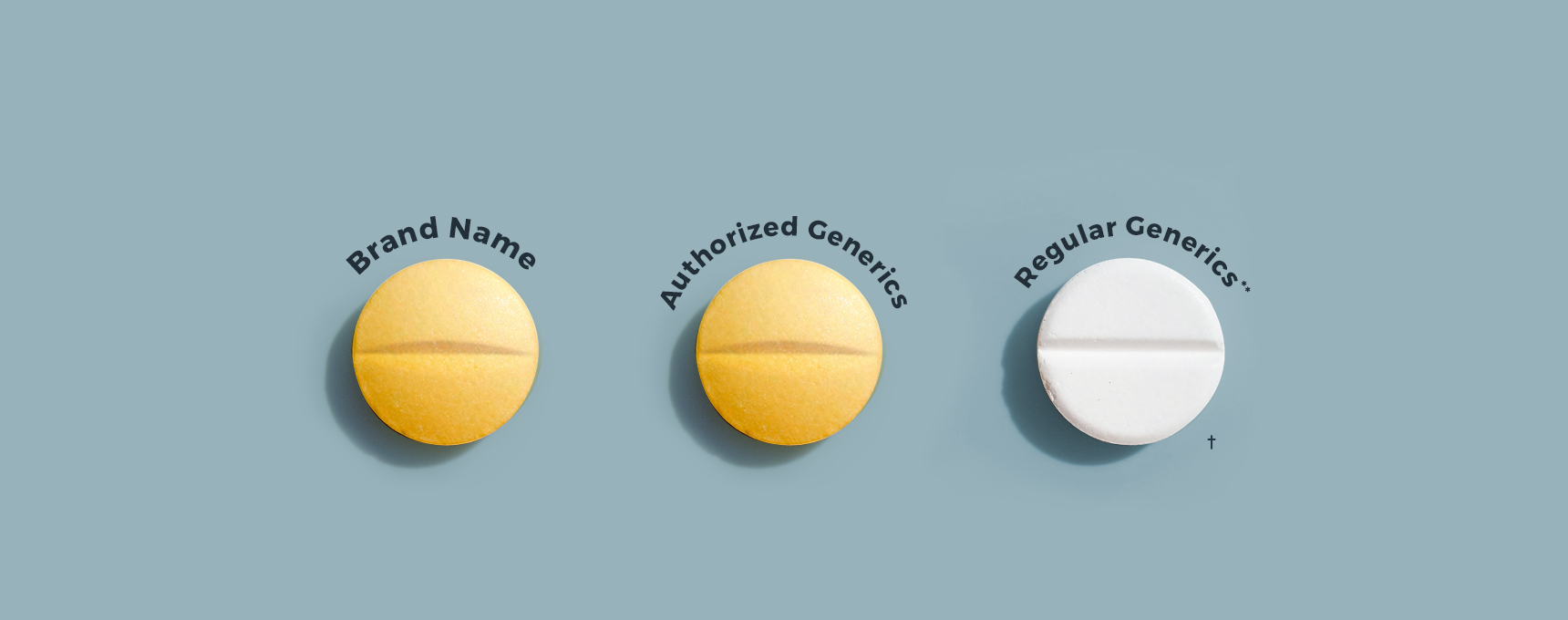
** Drugs commonly referred to as generics approved by the FDA under Abbreviated New Drug Applications (ANDAs).
† Not actual size. Not actual drugs sold here. Images are for illustrative purposes only.
Authorized Generics: the difference
Generic versions of brand-name drugs represent approximately 90% of all prescriptions. Many people don’t realize there are two kinds of generic drugs: Authorized Generics and Regular Generics.* Both an authorized generic and regular generic are: a) approved by the U.S. Food and Drug Administration (FDA); b) therapeutically equivalent to the corresponding brand-name drug; and c) as safe and effective as the brand. However, there are differences between authorized generics and regular generics.
When compared to the Brand Drug |
Authorized Generics |
Regular Generics |
|---|---|---|
|
Active Ingredient Strength & Dosage
Authorized generics and regular generics are both identical with respect to the active pharmaceutical ingredients, dosage form, strength, and route of administration.1 |
|
|
|
FDA Approval
Authorized generics exist under the same New Drug Application (NDA) as the Reference Listed Drug (also known as the brand). Regular generics are approved by the FDA under an Abbreviated New Drug Application (ANDA), which requires a regular generic to demonstrate bioequivalence to the corresponding brand product. |
|
Different* |
|
Manufacturing
Most authorized generics are made in the same exact manufacturing facility as the brand-name drugs and to the same specifications as the brand products. Generally, each regular generic is made in a different facility by a different company. The generic manufacturer does not have access to the brand’s proprietary manufacturing process. |
|
Different* |
|
Active Ingredient Availability at the Site of Drug Action
As part of the regular generics approval process, regular generics must demonstrate that they are bioequivalent to the brand product, meaning the rate and extent to which the active ingredient becomes available at the site of drug action is not significantly different from the brand product. The FDA criteria for bioequivalence allows for some differences vs. the NDA product within the parameters established by the FDA.2 |
|
Not significantly different |
|
Inactive Ingredients
Regular generic inactive ingredients may be different than the brand.1 |
|
May be different* |
|
Taste, Smell, Mouthfeel
Regular generics can have a different taste, smell, or feel in the mouth (for example, the coating of the generic may be different from the brand drug).1 |
|
May be different* |
|
Size & Shape
Regular generics can have significant variations in size and shape. Regular generic drug companies may produce different sizes and shapes of the same drug, so one generic company’s drug has a different size and shape compared to another generic company’s drug. Authorized generics are generally identical to the brand; however, there may be differences in markings, colors, labeling, and packaging. |
|
May be different* |
You have a choice when it comes to prescription medications
Learn more about FDA-approved authorized generics.
* Regular generic drugs are allowed (but not required) to differ from the brand drug in this respect.
1 U.S. Food and Drug Administration. FDA List of Authorized Generic Drugs
2 U.S. Food and Drug Administration. Guidance for Industry